Malaria, New Malaria Vaccine R21/MatrixM
Subscribe to Never Miss an Important Update! Assured Discounts on New Products!
Must Join PMF IAS Telegram Channel & PMF IAS History Telegram Channel
- Context (TH | TH | IE | FP | WIRE): The WHO recommended (but yet to be prequalified) a malaria vaccine, R21/MatrixM.
|
Malaria
- Malaria is a mosquito-borne infectious disease that affects humans and other animals.
- It is caused by single-celled microorganisms of the Plasmodium group of protozoans (microscopic heterotrophs that live as predators or parasites).

- Five Plasmodium species cause malaria in humans.
- Plasmodium falciparum and Plasmodium vivax pose the greatest threat.
- Recent evidence has shown drug-resistant mutations in Plasmodium Falciparum.
- P. ovale and P. malariae generally cause a milder form of malaria.
- P. knowlesi rarely causes disease in humans.
- Transmission: Infected female Anopheles mosquitoes transmit Plasmodium parasites through bites. The parasites multiply in the liver and destroy red blood cells (RBCs).
- Symptoms: Fever, chills, yellow skin, seizures etc.
- Treatment: It is preventable as well as curable. Prevention of malaria includes medications and mosquito elimination.
- Distribution: It is mostly found in tropical and subtropical areas of Africa, Asia, and South America.
|
Global Scenario of Malaria and Why Malaria Vaccine for Childeren is Required
- According to WHO, in 2021, worldwide there were 247 million malaria cases and 6,19,000 deaths.
- African Region accounted for 95% of all malaria cases and 96% of all malaria deaths in 2021.
- Children under age five accounted for about 80% of all malaria deaths in the African Region.
- Moreover, 25 million children are born yearly in countries with moderate to high malaria transmission.
Challenges to Malaria Control and Elimination
|
About R21/MatrixM: The New Malaria Vaccine

- It is the world’s second WHO-recommended malaria vaccine for children, after RTS,S in 2021.
- It is developed by Oxford University and manufactured by the Serum Institute of India (SII).
- The new vaccine will be sold under the brand Mosquirix.
- It has been approved for use in Burkina Faso, Ghana, and Nigeria for children under 36 months.
- This vaccine is meant for children under the age of five years
- It has three primary doses and a booster shot after a year.
- It is specific to Plasmodium falciparum. So, it cannot be used to prevent infections caused by other malaria parasites like Plasmodium vivax.
- It uses the same adjuvant, Matrix M, as the COVID-19 vaccine, Novavax, a version of which was also rolled out by SII.
|
Benefits of the R21 Malaria Vaccine:
- High efficacy: It has an efficacy of 75% in areas with seasonal prevalence and 68% in areas where the disease circulates all year round.
- Low cost: It is about half the price of RTS,S, the only other malaria vaccine available.
- Mass production: Serum Institute of India has the potential to mass-produce it on a large scale.
Efficacy of R21 Malaria Vaccine
- The vaccine efficacy was maintained for 18 months, with a single booster dose given 12 months after the primary series.
- Efficacy is higher in younger children (5-17 months) than older ones (18-36 months), suggesting potential lower effectiveness in those previously exposed to malaria.
- Also, the vaccine was more efficacious in seasonal malaria areas than in perennial ones. This suggests the vaccine will be not highly effective for children in areas with very high malaria incidence.
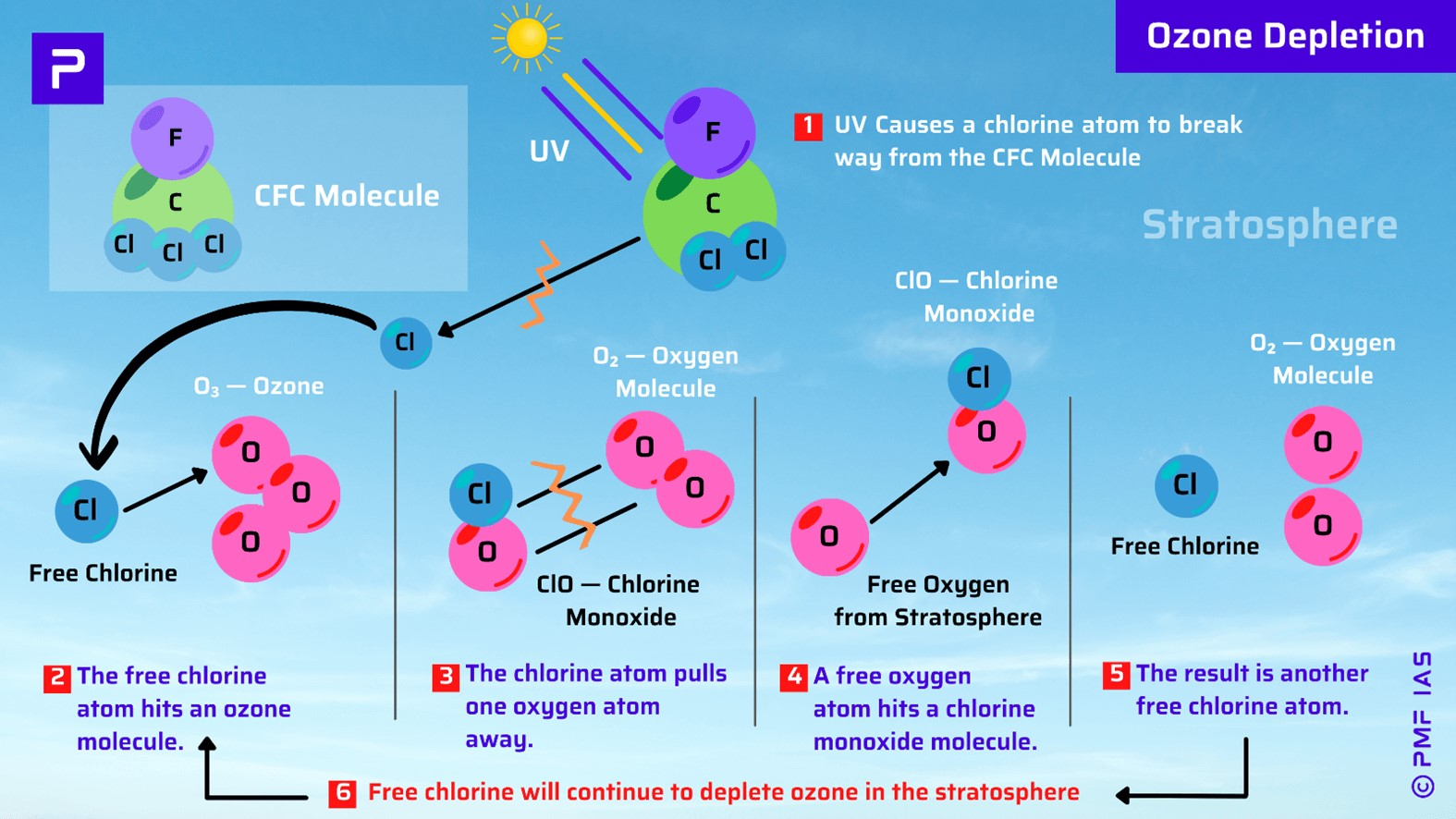
How the Malaria Vaccines Works?
- Vaccines are designed to stimulate the immune system against specific infectious diseases.
- Both the newly endorsed malaria vaccines, R21 and RTS,S, are sub-unit vaccines that aim to protect against malaria caused by Plasmodium falciparum parasite.
- They contain the circumsporozoite protein, which coats the surface of the P. falciparum parasite.
- The protein on the vaccines triggers an immune response against the P. falciparum parasite
- It’s a bit like how most COVID vaccines target the “spike” protein on the surface of SARS-CoV-2 (the virus that causes COVID) to block the virus.
|
|
Challenges in Eradication of Malaria in Children
- Administration of booster shots: The trials show that yearly boosters will be required to maintain protection. But boosters are challenging to administer even in areas with robust health systems.
- Non-integration with children vaccination programmes: To date, neither malaria vaccine has been integrated into other childhood vaccination programs, placing an additional burden on communities.
- Very high malaria-risk areas: There is a risk that previous malaria infection can block the efficacy of vaccines, leaving those most at risk with reduced protection.
Need of Malaria Vaccine for India
- Context (TH): Both the endorsed malaria vaccines does not target the malaria in India which is caused by Plasmodium vivax parasites.
- They target only the malaria caused by Plasmodium falciparum parasites which is the deadliest.
Malaria Scenario in India
- India saw an 85.1% decrease in malaria cases and an 83.36% drop in deaths from 2015 to 2022.
- However, according to the WHO, India accounts for 83% of malaria cases in southeast Asia.
- India is now in a lower-endemic area, reporting fewer than 100 deaths in the last two to three years.
Does India Need a Malaria Vaccine?
- Before rolling out any vaccine or any programme, we must do a cost-benefit analysis.
- WHO has done this assessment only in high burden countries where vaccine is cost-effective.
- In low-burden countries like India, vaccination is not very cost-effective.
- Moreover, Plasmodium Vivax is not a very severe variant.
Way Forward
- Many countries like Sri Lanka, Maldives and China, which were high burden countries, eliminated malaria only by control measures.
- There are various control measures under the National Vector Disease Control programme need to be accelerated.
Way Ahead
Malaria Vaccines with Wider Coverage
- Both endorsed vaccines only target one type of malaria, that is, caused by P. falciparum parasites. It’s the deadliest form of malaria, so vaccine efforts have primarily targeted this species.
- These vaccines don’t protect against other malaria types, esp. P. vivax, a major concern in SE Asia.
- So, it is essential to develop second-generation malaria vaccines, which can:
- Induce high levels of sustained protection without the need for yearly boosters
- Protect against all malaria types (particularly P. vivax)
- Protect children who have the highest malaria risk




![PMF IAS Environment for UPSC 2022-23 [paperback] PMF IAS [Nov 30, 2021]…](https://pmfias.b-cdn.net/wp-content/uploads/2024/04/pmfiasenvironmentforupsc2022-23paperbackpmfiasnov302021.jpg)