Carbohydrates | Monosaccharides | Polysaccharides
Subscribe to Never Miss an Important Update! Assured Discounts on New Products!
Must Join PMF IAS Telegram Channel & PMF IAS History Telegram Channel
Biomolecules – Carbohydrates – Monosaccharides: Glucose, Fructose; Disaccharides: Sucrose, Lactose; Oligosaccharides and Polysaccharides: Starch, Cellulose, Glycogen.
Compiled from NCERT Science Textbooks Class 6-12.
Video Explanation:
Biomolecule
- A biomolecule [biological molecule] is any molecule that is present in living organisms –– microorganisms, plants and animals.
- They are mostly made up of carbon, oxygen, hydrogen and nitrogen.
- Proteins, carbohydrates, lipids, and nucleic acids [DNA and RNA] are Macromolecules or Macro-biomolecules.
- Other small molecules such as vitamins, primary metabolites, secondary metabolites, etc. are also biomolecules.
- Most biomolecules are organic compounds.
Metabolism == the chemical processes that occur within a living organism to maintain life.
Metabolite == a substance formed in or necessary for metabolism.
Primary metabolite == Metabolite that is directly involved in normal growth, development, and reproduction. Eg: ethanol, lactic acid, and certain amino acids.
Secondary metabolite == Metabolites that are not directly involved in the normal growth, development, or reproduction of an organism. Unlike primary metabolites, absence of secondary metabolites does not result in immediate death, but rather in long-term impairment. Eg: ergot alkaloids, antibiotics, etc.
Alkaloid == any of a class of nitrogenous organic compounds of plant origin which have pronounced physiological actions on humans. Eg: morphine obtained from opium poppy.
Carbohydrates
- Carbohydrates are one of the most important biomolecules that forms a major part of the living things.
- Carbohydrates are primarily produced by plants and form a very large group of naturally occurring organic compounds.
- Some common examples of carbohydrates are cane sugar, glucose, starch,
- Most of them have a general formula, Cx(H2O)y, and were considered as hydrates of carbon from where the name carbohydrate was derived.
Hydrate == a compound in which water molecules are chemically bound to another compound or an element. Eg: α-d-Glucose hydrate (C6H14O7).
- For example, the molecular formula of glucose (C6H12O6) fits into this general formula, C6(H2O)6. But all the compounds which fit into this formula may not be classified as carbohydrates.
Acetic acid (CH3COOH) fits into this general formula Cx(H2O)y → C2(H2O)2 but is not a carbohydrate.
Exception: Rhamnose, C6H12O5 is a carbohydrate but does not fit in this definition of Cx(H2O)y.
- Chemically, the carbohydrates may be defined as optically active polyhydroxy [multiple HO groups] aldehydes or ketones or the compounds which produce such units on hydrolysis.
Carbohydrates produce aldehydes and ketones on hydrolysis [the chemical breakdown of a compound due to reaction with water].
Aldehyde == an organic compound containing the group — CHO, formed by the oxidation of alcohols. Typical aldehydes include methanal (formaldehyde) and ethanal (acetaldehyde).
Ketone == an organic compound containing a carbonyl group =C=O bonded to two alkyl groups, e.g. acetone].
Alkyl == denoting a hydrocarbon radical derived from an alkane by removal of a hydrogen atom].
Alkane == any of the series of saturated hydrocarbons including methane, ethane, propane, and higher members].
- Some of the carbohydrates, which are sweet in taste, are also called sugars.
- The most common sugar, used in our homes is named as sucrose whereas the sugar present in milk is known as lactose.
- Carbohydrates are also called saccharides (Greek: sakcharon means sugar).
- Carbohydrates are classified on the basis of their behavior on hydrolysis. They have been broadly divided into following three groups.
Monosaccharides
- A carbohydrate that cannot be hydrolyzed further to give simpler unit of polyhydroxy aldehyde or ketone is called a monosaccharide.
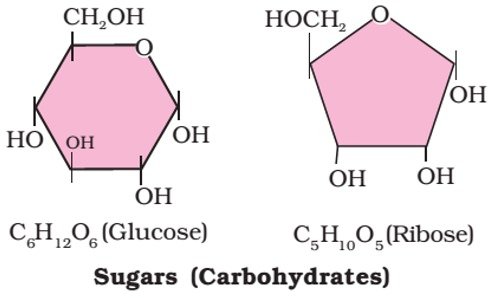
- About 20 monosaccharides are known to occur in nature. Some common examples are Glucose, Fructose, Ribose, Galactose, etc.
- If a monosaccharide contains an aldehyde group [–CHO], it is known as an aldose and if it contains a keto group [=C=O], it is known as a ketose.
Glucose
- Glucose occurs freely in nature as well as in the combined form.
- It is present in sweet fruits and honey. Ripe grapes also contain glucose in large amounts.
- Glucose is an aldohexose [An aldohexose is a hexose with an aldehyde group on one end] and is also known as dextrose. It is the monomer of many of the larger carbohydrates, namely starch, cellulose.
Aldohexose == An aldohexose is a hexose with an aldehyde group on one end.
Aldehyde group [–CHO]
Hexose == any of the class of simple sugars whose molecules contain six carbon atoms (e.g. glucose)
- It is probably the most abundant organic compound on earth.
- Glucose is found to exist in two different crystalline forms which are named as α and β.
- Such isomers, i.e., α-form and β-form, are called anomers.
Fructose
- Fructose is an important ketohexose. It is obtained along with glucose by the hydrolysis of disaccharide, sucrose.
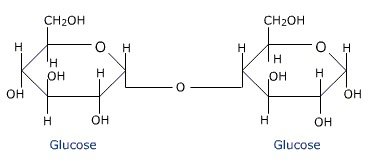
- The two monosaccharides are joined together by an oxide linkage formed by the loss of a water molecule.
- Such a linkage between two monosaccharide units through oxygen atom is called Glycosidic Linkage.
Ribose
- The ribose β-D-ribofuranose forms part of the backbone of RNA. It is related to deoxyribose, which is found in DNA.
Galactose
- Galactose is a monosaccharide. When combined with glucose (monosaccharide), through a condensation reaction, the result is the disaccharide lactose.
- The hydrolysis of lactose to glucose and galactose is catalyzed by the enzymes lactase and β-galactosidase.
Oligosaccharides
- Carbohydrates that yield two to ten monosaccharide units, on hydrolysis, are called oligosaccharides.
- They are further classified as disaccharides, trisaccharides, tetrasaccharides, etc., depending upon the number of monosaccharides, they provide on hydrolysis.
- Amongst these the most common are disaccharides.
- The two monosaccharide units obtained on hydrolysis of a disaccharide may be same or different.
- For example, sucrose on hydrolysis gives one molecule each of glucose and fructose whereas maltose gives two molecules of glucose only.
Sucrose == Glucose + Fructose
Maltose == Glucose + Glucose
Lactose == Glucose + Galactose
Sucrose
- One of the common disaccharides is sucrose which on hydrolysis gives equimolar mixture of glucose and fructose.
Maltose
- Another disaccharide, maltose is composed of two α-D-glucose units
Lactose
- It is more commonly known as milk sugar since this disaccharide is found in milk. It is composed of β-D-galactose and β-D-glucose.
Polysaccharides
- Carbohydrates which yield a large number of monosaccharide units on hydrolysis are called polysaccharides.
- Some common examples are Starch, Cellulose, Glycogen, Gums,
- Polysaccharides are long chains of sugars. Polysaccharides are not sweet in taste, hence they are also called non-sugars.
- They are threads (literally a cotton thread) containing different monosaccharides as building blocks.
- For example, Cellulose is a polymeric polysaccharide consisting of only one type of monosaccharide i.e., Glucose. Cellulose is a homopolymer. Starch is a variant of this but present as a store house of energy in plant tissues.
- Animals have another variant called Glycogen.
- Inulin is a polymer of fructose.
- Plant cell walls are made of cellulose. Paper made from plant pulp and cotton fibre is cellulosic. There are more complex polysaccharides in nature.
- Exoskeletons of arthropods, for example, have a complex polysaccharide called These complex polysaccharides are mostly homopolymers.
Starch
- Polysaccharides contain a large number of monosaccharide units joined together by glycosidic linkages.
- These are the most commonly encountered carbohydrates in nature.
- They mainly act as the food storage or structural materials.
- Starch is the main storage polysaccharide of plants.
- It is the most important dietary source for human beings.
- High content of starch is found in cereals, roots, tubers and some vegetables.
- It is a polymer of α-glucose and consists of two components — Amylose and Amylopectin.
- Amylose is water soluble polysaccharide which constitutes about 15-20% of starch.
- Amylopectin is water insoluble polysaccharide which constitutes about 80- 85% of starch.
Cellulose
- Cellulose occurs exclusively in plants and it is the most abundant organic substance in plant kingdom.
- It is a predominant constituent of cell wall of plant cells.
- Cellulose is a straight chain polysaccharide composed only of β-D-glucose units.
Glycogen
- The carbohydrates are stored in animal body as
- It is also known as animal starch because its structure is similar to amylopectin and is rather more highly branched.
- It is present in liver, muscles and brain.
- Glycogen is also found in yeast and fungi.
- When the body needs glucose, enzymes break the glycogen down to glucose.
Importance of Carbohydrates
- Carbohydrates are essential for life in both plants and animals.
- They form a major portion of our food. Honey has been used for a long time as an instant source of energy in ayurvedic system of medicine.
- Carbohydrates are used as storage molecules as starch in plants and glycogen in animals.
- Cell wall of bacteria and plants is made up of cellulose which is a carbohydrate.
- We build furniture, etc. from cellulose in the form of wood and clothe ourselves with cellulose in the form of cotton fibre.
- They provide raw materials for many important industries like textiles, paper, lacquers and breweries.




![PMF IAS Environment for UPSC 2022-23 [paperback] PMF IAS [Nov 30, 2021]…](https://pmfias.b-cdn.net/wp-content/uploads/2024/04/pmfiasenvironmentforupsc2022-23paperbackpmfiasnov302021.jpg)