Proteins | Amino Acids | Enzymes
Subscribe to Never Miss an Important Update! Assured Discounts on New Products!
Must Join PMF IAS Telegram Channel & PMF IAS History Telegram Channel
General Science for UPSC: Amino Acids – Proteins – Structure of Proteins, Fibrous proteins, Globular proteins, Role of Proteins. Enzymes, Factors Affecting Enzyme Activity.
Compiled from NCERT Science Textbooks Class 6-12.
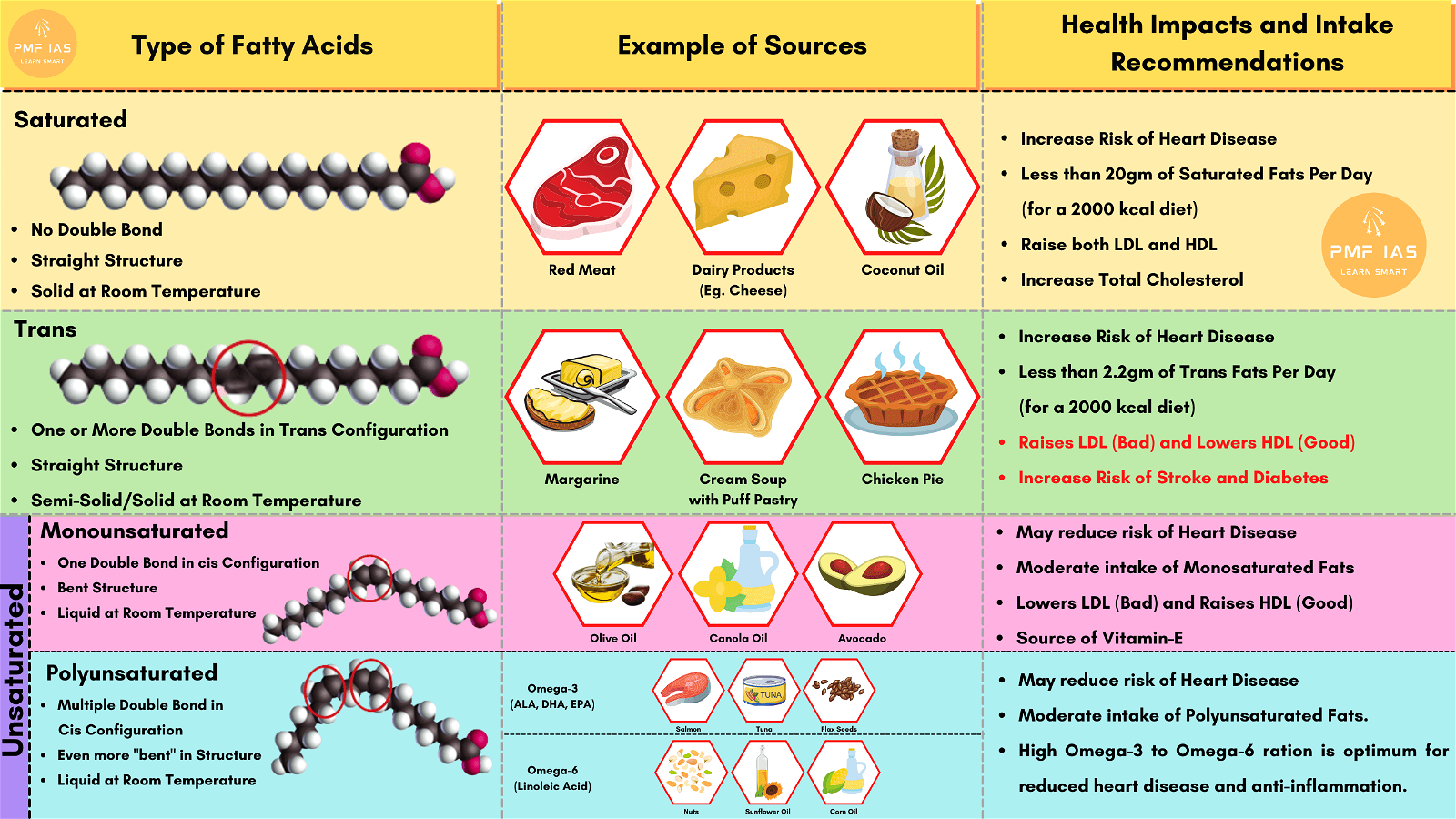
Amino Acids
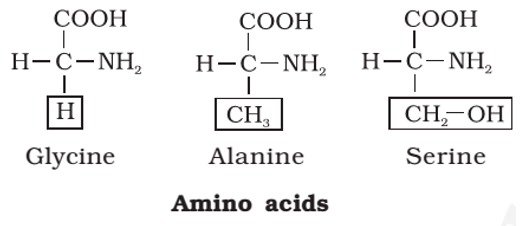
- Amino acids are organic compounds containing an amino group [NH2] and an acidic group [COOH] as substituents on the same carbon i.e., the a-carbon. Hence, they are called a-amino acids. They are substituted methanes.
- All proteins are polymers of α-amino acids.
- Amino acids contain amino (–NH2) and carboxyl (–COOH) functional groups.
- Depending upon the relative position of amino group with respect to carboxyl group, the amino acids can be classified as α, β, γ, δ and so on.
- Only α-amino acids are obtained on hydrolysis of proteins.
- All α-amino acids have trivial names, which usually reflect the property of that compound or its source.
- Glycine is so named since it has sweet taste (in Greek glykos means sweet) and tyrosine was first obtained from cheese (in Greek, tyros means cheese.)
- Amino acids are classified as acidic, basic or neutral depending upon the relative number of amino and carboxyl groups in their molecule.
- Equal number of amino and carboxyl groups makes it neutral;
- more number of amino than carboxyl groups makes it basic and
- more carboxyl groups as compared to amino groups makes it acidic.
- The amino acids, which can be synthesized in the body, are known as nonessential amino acids.
- On the other hand, those which cannot be synthesized in the body and must be obtained through diet, are known as essential amino acids.
- Amino acids are usually colorless, crystalline solids. These are water-soluble, high melting solids and behave like salts rather than simple amines or carboxylic acids.
- This behavior is due to the presence of both acidic (carboxyl group) and basic (amino group) groups in the same molecule.
- In aqueous solution, the carboxyl group can lose a proton and amino group can accept a proton, giving rise to a dipolar ion known as zwitter ion. This is neutral but contains both positive and negative charges.
- In zwitter ionic form, amino acids show amphoteric behavior as they react both with acids and bases.
- Except glycine, all other naturally occurring α-amino acids are optically active, since the α-carbon atom is asymmetric.
Optically Active: https://www.youtube.com/watch?v=gBELxxGbzKk
Proteins
- Proteins are the most abundant biomolecules of the living system.
- Chief sources of proteins are milk, cheese, pulses, peanuts, fish, meat, etc.
- They occur in every part of the body and form the fundamental basis of structure and functions of life.
- They are also required for growth and maintenance of body.
- The word protein is derived from Greek word, “proteios” which means primary or of prime importance.
- Proteins are polypeptides.
[Peptide == a compound consisting of two or more amino acids linked in a chain].
- Proteins are linear chains of amino acids linked by peptide bonds.
- Each protein is a polymer of amino acids.
[Monomer == a molecule that can be bonded to other identical molecules to form a polymer].
- Dietary proteins are the source of essential amino acids.
- Therefore, amino acids can be essential or non-essential.
[Non-Essential Amino Acids == Amino Acids that our body can make].
[Essential Amino Acids == We get them through our diet/food].
- Collagen is the most abundant protein in animal world.
- Ribulose bisphosphate Carboxylase-Oxygenase (RuBisCO) is the most abundant protein in the whole of the biosphere.
Structure of Proteins
- You have already read that proteins are the polymers of α-amino acids and they are connected to each other by peptide bond or peptide linkage.
- Chemically, peptide linkage is an amide [an organic compound containing the group -C(O)NH2] formed between –COOH group and –NH2
- The reaction between two molecules of similar or different amino acids, proceeds through the combination of the amino group of one molecule with the carboxyl group of the other.
- This results in the elimination of a water molecule and formation of a peptide bond –CO–NH–. The product of the reaction is called a dipeptide because it is made up of two amino acids.
- If a third amino acid combines to a dipeptide, the product is called a tripeptide.
- A tripeptide contains three amino acids linked by two peptide linkages.
- Similarly when four, five or six amino acids are linked, the respective products are known as tetrapeptide, pentapeptide or hexapeptide, respectively.
- When the number of such amino acids is more than ten, then the products are called polypeptides.
- A polypeptide with more than hundred amino acid residues, having molecular mass higher than 10,000u is called a protein.
- However, the distinction between a polypeptide and a protein is not very sharp.
- Polypeptides with fewer amino acids are likely to be called proteins if they ordinarily have a well-defined conformation of a protein such as insulin which contains 51 amino acids.
- Proteins can be classified into two types on the basis of their molecular shape: Fibrous Proteins and Globular proteins.
Fibrous proteins
- When the polypeptide chains run parallel and are held together by hydrogen and disulphide bonds, then fibre– like structure is formed.
- Such proteins are generally insoluble in water. Some common examples are keratin (present in hair, wool, silk) and myosin (present in muscles), etc.
Globular proteins
- This structure results when the chains of polypeptides coil around to give a spherical shape.
- These are usually soluble in water. Insulin and albumins are the common examples of globular proteins.
Primary structure of proteins
- Proteins may have one or more polypeptide chains. Each polypeptide in a protein has amino acids linked with each other in a specific sequence and it is this sequence of amino acids that is said to be the primary structure of that protein.
- Any change in this primary structure i.e., the sequence of amino acids creates a different protein.
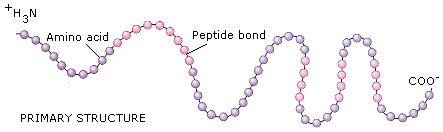
Secondary structure of proteins
- The secondary structure of protein refers to the shape in which a long polypeptide chain can exist.
- Protein found in a biological system with a unique three-dimensional structure and biological activity is called a native protein.
- When a protein in its native form, is subjected to physical change like change in temperature or chemical change like change in pH, the hydrogen bonds are disturbed. Due to this, globules unfold and helix get uncoiled and protein loses its biological activity. This is called denaturation of protein.
- During denaturation 2° and 3° structures are destroyed but 1º structure remains intact. The coagulation of egg white on boiling is a common example of denaturation. Another example is curdling of milk which is caused due to the formation of lactic acid by the bacteria present in milk.
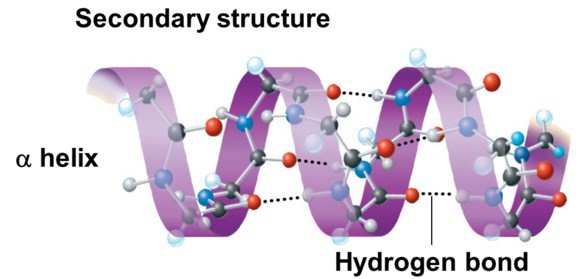
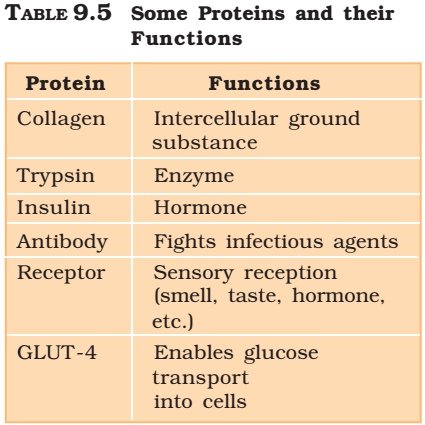
Role of Proteins
- Some transport nutrients across cell membrane,
- some fight infectious organisms,
- some are hormones,
- some are enzymes, etc.
Enzymes
- Life is possible due to the coordination of various chemical reactions in living organisms. An example is the digestion of food, absorption of appropriate molecules and ultimately production of energy. This process involves a sequence of reactions and all these reactions occur in the body under very mild conditions. This occurs with the help of certain biocatalysts called enzymes.
Catalyst == a substance that increases the rate of a chemical reaction without itself undergoing any permanent chemical change.
- Almost all the enzymes are globular proteins.
- Enzymes are very specific for a particular reaction and for a particular substrate.
- They are generally named after the compound or class of compounds upon which they work. For example, the enzyme that catalyses hydrolysis of maltose into glucose is named as maltase.
- Sometimes enzymes are also named after the reaction, where they are used. For example, the enzymes which catalyse the oxidation of one substrate with simultaneous reduction of another substrate are named as oxidoreductase The ending of the name of an enzyme is -ase.
- Almost all enzymes are proteins.
- There are some nucleic acids that behave like enzymes. These are called ribozymes.
- An enzyme like any protein has a primary structure, i.e., amino acid sequence of the protein.
- Enzyme catalysts differ from inorganic catalysts in many ways. Inorganic catalysts work efficiently at high temperatures and high pressures, while enzymes get damaged at high temperatures (say above 40°C).
- However, enzymes isolated from organisms who normally live under extremely high temperatures (e.g., hot vents and sulphur springs), are stable and retain their catalytic power even at high temperatures (up to 80°-90°C). Thermal stability is thus an important quality of such enzymes isolated from thermophilic organisms.
Thermophile == a bacterium or other microorganism that grows best at high temperatures (above 45°C).
Factors Affecting Enzyme Activity
- The activity of an enzyme can be affected by a change in the conditions which can alter the structure of the protein. These include temperature, pH, change in substrate concentration or binding of specific chemicals that regulate its activity.
Temperature and pH
- Enzymes generally function in a narrow range of temperature and pH.
- Each enzyme shows its highest activity at a particular temperature and pH called the optimum temperature and optimum pH.
- Activity declines both below and above the optimum value.
- Low temperature preserves the enzyme in a temporarily inactive state whereas high temperature destroys enzymatic activity because proteins are denatured by heat.
Concentration of Substrate
- With the increase in substrate concentration, the velocity of the enzymatic reaction rises at first. The reaction ultimately reaches a maximum velocity (Vmax) which is not exceeded by any further rise in concentration of the substrate. This is because the enzyme molecules are fewer than the substrate molecules and after saturation of these molecules, there are no free enzyme molecules to bind with the additional substrate molecules.
- The activity of an enzyme is also sensitive to the presence of specific chemicals that bind to the enzyme. When the binding of the chemical shuts off enzyme activity, the process is called inhibition and the chemical is called an inhibitor.
- When the inhibitor closely resembles the substrate in its molecular structure and inhibits the activity of the enzyme, it is known as competitive inhibitor.
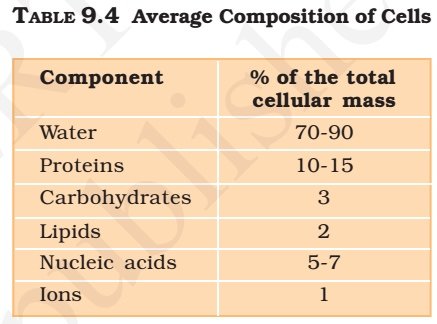
Summary
- Proteins are the polymers of about twenty different α-amino acids which are linked by peptide bonds.
- Ten amino acids are called essential amino acids because they cannot be synthesised by our body, hence must be provided through diet.
- Proteins perform various structural and dynamic functions in the organisms.
- Proteins which contain only α-amino acids are called simple proteins.
- The secondary or tertiary structure of proteins get disturbed on change of pH or temperature and they are not able to perform their functions. This is called denaturation of proteins.
- Enzymes are biocatalysts which speed up the reactions in biosystems. They are very specific and selective in their action and chemically all enzymes are proteins.




![PMF IAS Environment for UPSC 2022-23 [paperback] PMF IAS [Nov 30, 2021]…](https://pmfias.b-cdn.net/wp-content/uploads/2024/04/pmfiasenvironmentforupsc2022-23paperbackpmfiasnov302021.jpg)