Iron Ore Distribution across the World
Subscribe to Never Miss an Important Update! Assured Discounts on New Products!
Must Join PMF IAS Telegram Channel & PMF IAS History Telegram Channel
Iron Ore – Raw Material, Impurities in Iron Ore, What exactly happens in a blast furnace? Smelting, Beneficiation. Iron Ore Distribution Across the World.
Factors that influence the location of Iron and Steel industry
- Raw materials – iron ore, coal, limestone, etc.
- Transportation and other infrastructure – road, rail, ports etc.
- Investment and Entrepreneurship = banking facilities, human capital for managerial roles.
- Labour – unskilled to semi-skilled workforce for manual operations, skilled workforce for technical operations.
- Market – construction industry, automobile industry etc.
- Government policy – Development agenda, land acquisition, ease of doing business = labor laws, unambiguous and fair taxation policy, least government interference, less red tapeism, quick environmental clearance [Read more from: www.mrunal.org/geography].
Iron Ore – Raw Material
The below data is important for Prelims [Will be helpful to answer some logic based questions in mains]
- To understand about the factors that influence the location of Iron and Steel Industry, we have to understand about iron ore smelting.
- Smelting is a process of converting ore to metal by removing impurities.
Commonly found impurities in Iron Ore
Silicon
- Found in small quantities.
- Slightly raises the Strength and Hardness of Steel.
- Acts as a de-oxidizing Agent ==> small quantities is good. [Oxides decrease the strength of Iron]
Sulphur
- A VERY harmful element.
- Forms Iron Sulphide which is a very brittle
- Greatly reducing the Strength of Steel ==> very bad.
Phosphorous
- Combines with Iron to form a Phosphide.
- It increases the hardness and Tensile strength of Steel.
- It SERIOUSLY affects the ductility and resistance to shock or impact ==> bad.
Lead
- Added to all classes of Steel to improve the machinability of the Steel.
- It improves tool life ==> small quantities is good.
Manganese
- A powerful and most effective de-oxidant.
- Has a good effect on Sulphur ==> small quantities is good.
Tin
- It forms a low melting point brittle film round the grain boundaries making the Steel practically useless ==> very bad.
Oxygen
- Has a bad influence on the properties of steel ==> very bad. [Oxides make Iron and steel weak]
Of the impurities, some are beneficial when present in small quantities while the others are harmful no matter what their proportion is.
So, the unwanted impurities must be removed and this is done by smelting iron ore in a blast furnace.
What exactly happens in a blast furnace?
- In a blast furnace, fuel (coke), iron ore, and flux (limestone) are continuously supplied through the top of the furnace.
- A hot blast of air (sometimes with oxygen enrichment) is blown into the lower section.
- In a blast furnace, iron oxides are converted into liquid iron called “hot metal”.
[Oxides make iron brittle. To make iron strong the oxides need to be removed]
Inputs in to blast furnace
- Ore == iron ore,
- Fuel == coke,
- Flux == limestone,
Output
- Final product è liquid slag, liquid iron (pig iron) and gases.
Beneficiation = Improve Concentration of Iron
- Ore is either Hematite (Fe2O3) or Magnetite (Fe3O4) and the iron content ranges from 50% to 70%.
- This iron rich ore can be charged directly into a blast furnace without any further processing.
- Iron ore that contains a lower iron content must be processed or beneficiated to increase its iron content.
[Beneficiation è Improves the concentration of iron ore]
Why coke and not coal in smelting?
- To separate impurities, iron needs to be melted.
- The coke is the fuel that melts iron.
- Coal has many impurities and the most dangerous one is sulphur.
- Coal is cooked to produce coke. This process is called destructive distillation.
- Coke is a fuel with few impurities and a high carbon content.
- The cooked coal, called coke contains 90 to 93% carbon, some ash and sulfur but compared to raw coal is very strong.
Role of limestone = Remove Sulphur
- It is acts as flux (a substance mixed with a solid to lower the melting point, especially in smelting).
- Limestone melts and reacts with Sulphur to form Slag (All solid and liquid impurities).
[Limestone marries Sulphur and takes it away from Iron == Very Good]
CaCO3 = CaO + CO2
The CaO formed from this reaction is used to remove sulfur from the iron.
FeS + CaO + C = CaS + FeO + CO
- The CaS [newly married couple] becomes part of the slag.
- The slag is also formed from any remaining Silica (SiO2), Alumina (Al2O3), Magnesia (MgO) or Calcia (CaO) that entered with the iron ore or coke.
- The liquid slag then trickles to the bottom of the furnace where it floats on top of the liquid iron since it is less dense.
Reduction = Remove Oxygen
- Oxygen in the iron oxides is reduced (removed) by a series of chemical reactions.
- 3Fe2O3 + CO = CO2 + 2Fe3O4
- Fe3O4 + CO = CO2 + 3 FeO
- FeO + CO = CO2 + Fe
CO or CARBON MONOXIDE is produced by burning coke.
So CO and CO2 are the gaseous pollutants coming out of blast furnace.
Pig Iron
- Pig iron is the intermediate product of smelting iron ore.
- Iron (Fe) = 93.5 – 95.0%
- Silicon (Si) = 0.30 – 0.90%
- Sulfur (S) = 0.025 – 0.050%
- Manganese (Mn) = 0.55 – 0.75%
- Phosphorus (P) = 0.03 – 0.09%
- Titanium (Ti) = 0.02 – 0.06%
- CARBON (C) = 4.1 – 4.4% [The strength of steel can be varied by varying the carbon content]
Cast iron
- Carbon content greater than 2%.
- Carbon (C) and silicon (Si) are the main alloying elements.
- Cast iron tends to be
- Applications: automotive industry parts, cast iron pan.
Steel
- Carbon content is up to 2.1% (by weight).
Stainless steel
- Steel alloy with a minimum of 5% chromium content by mass.
- Nickel is another important element of steel alloy.
- Also contains manganese, molybdenum, and other metals.
- Stainless steel does not readily corrode, rust or stain with water as ordinary steel does.
Wrought iron
- Cast iron assumes its finished shape the moment the liquid iron alloy cools down in the mold.
- Wrought iron is a very different material made by mixing liquid iron with some slag.
- The result is an iron alloy with a much lower carbon content.
- Wrought iron is softer than cast iron and much less tough, so you can heat it up to shape it relatively easily, and it’s also much less prone to rusting.
- Wrought iron is what people used to use before they really mastered making steel in large quantities in the mid-19th century.
Iron Ore Distribution Across the World
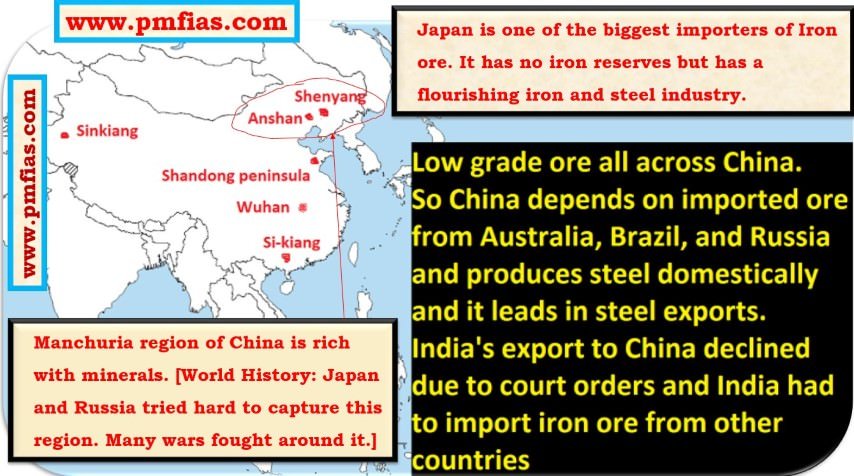
Iron Ore in China – Manchuria, Sinkiang, Si-kiang, Shandog Peninsula
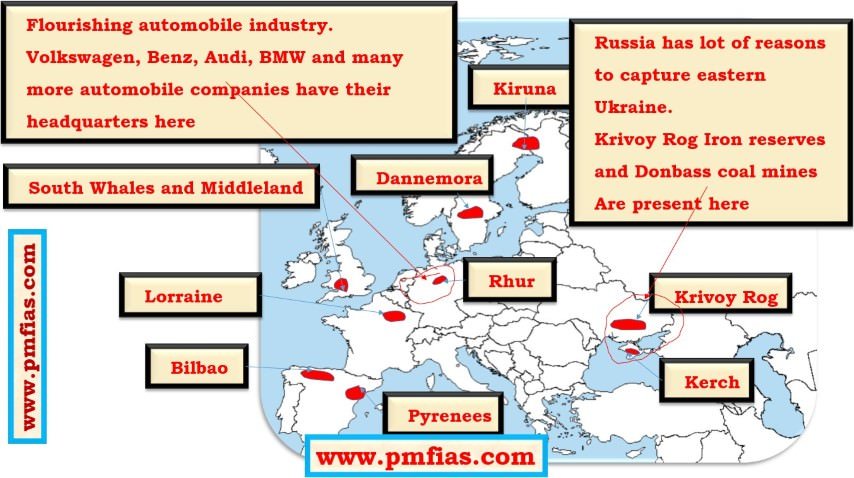
Iron Ore in Europe – Ruhr, South Whales, Krivoy Rog, Bilbao, Lorraine
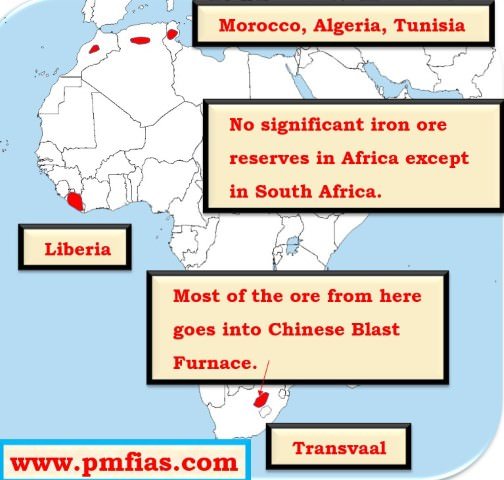
Iron ore in Africa – Transvaal, Liberia
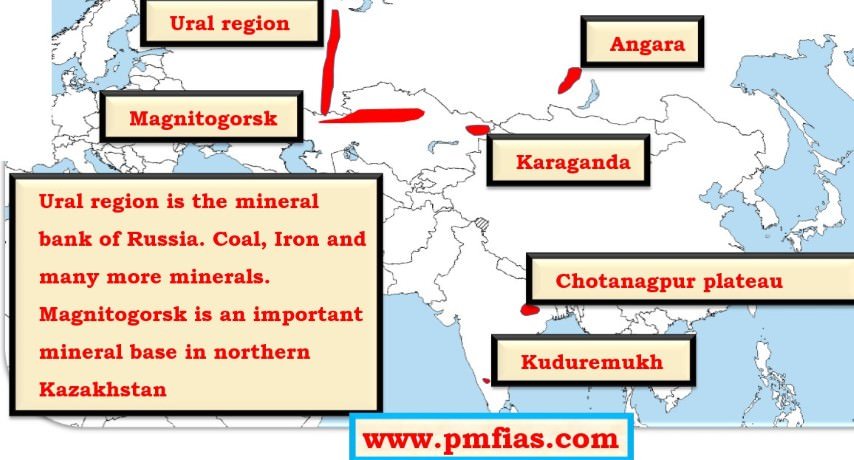
Iron ore in Russia, Kazakhstan – Ural region, Magnitogorsk
Iron Ore in North America – Great Lakes [Mesabi Region], Labrador
![Iron Ore in North America – Great Lakes [Mesabi Region], Labrador](http://pmfias.b-cdn.net/wp-content/uploads/2016/01/Iron-Ore-in-North-America-%E2%80%93-Great-Lakes-Mesabi-Region-Labrador-.jpg)
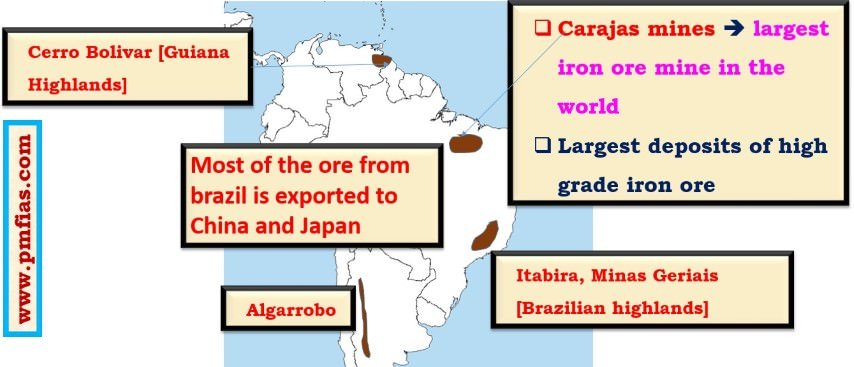
Iron Ore in South America – Carajas, Itabira, Minas Geriais
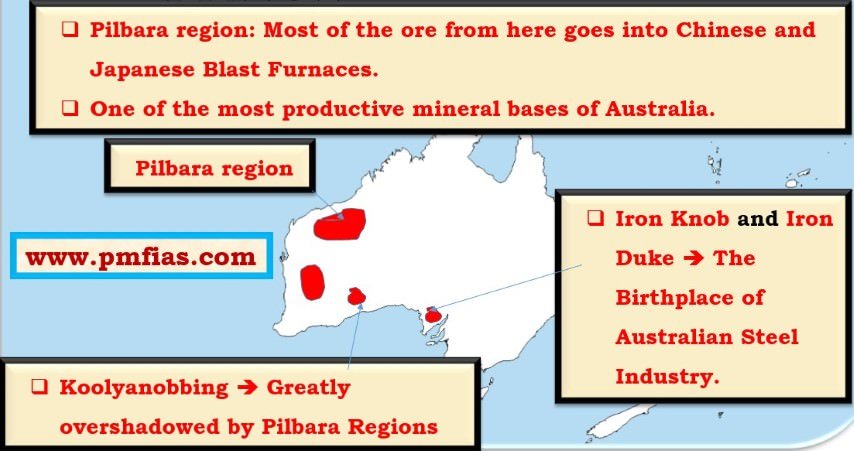
Iron Ore in Australia – Pilbara Region, Koolyanobbing, Iron Duke, Iron Knob
Primary References: NCERT Geography, Indian Geography by Kullar [Amazon and Flipkart]













What to say great job
why are you mentoned murnal website ? whether they are not good n geography
They do have good content on Geography and that is why I have mentioned it here.
Are these notes compilation of mrunal’s and your notes
No, these are mine. For Mrunal’s visit mrunal.org/geography
Hello team plz do reply
Wanted to know if doing ur notes alone is sufficient for geography , environment and science
I have taken all ur coursee
Do I still need to read ncert
kindly correct the map of India.
Magnitogorsk is in Russia. Kindly, rectify.
Eitherway, love the hand-written. It’s wayyy more interactive than animated world maps.
Regards,
Arpit
nodegreelearn.com