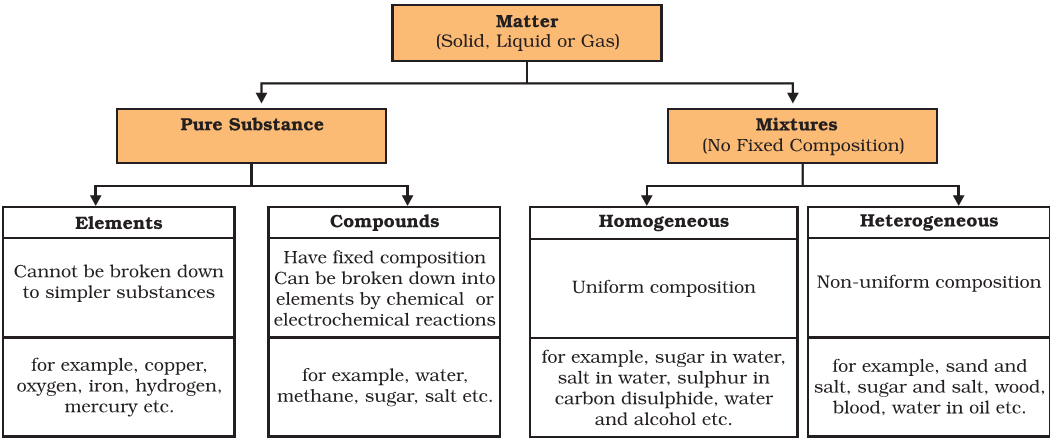
Elements, Metalloids, Compound, Mixtures vs. Compounds
Subscribe to Never Miss an Important Update! Assured Discounts on New Products!
Must Join PMF IAS Telegram Channel & PMF IAS History Telegram Channel
- Atoms of most elements are not able to exist independently.
- Atoms form molecules and ions.
- These molecules or ions aggregate in large numbers to form matter.
Source | Credits | Picture Credits: NCERT General Science
Elements
- Robert Boyle was the first scientist to use the term element in 1661.
- Antoine Lavoisier (1743-94) was the first to establish an experimentally useful definition of an element.
- He defined an element as a basic form of matter that cannot be broken down into simpler substances by chemical reactions.
- Element: A chemical element is a pure chemical substance consisting of a single type of atom distinguished by its atomic number, which is the number of protons in its atomic nucleus.
- If a substance cannot be broken down further by chemical reactions, by cooling, heating, or by electrolysis, it is called ‘element’. Sulphur is an element. So is iron. Carbon, too, is an element.
- Elements are divided into metals, metalloids, and non-metals.
- In the beginning, the names of elements were derived from the name of the place where they were found for the first time. For example, the name copper was taken from Cyprus.
- Some names were taken from specific colours. E.g. gold was taken from the English word meaning yellow.
- Now-a-days, IUPAC (International Union of Pure And Applied Chemistry) approves names of elements.
- Many of the symbols are the first one or two letters of the element’s name in English.
- The first letter of a symbol is always written as an uppercase and the second letter as a lowercase.
Some facts about elements
- The number of elements known at present are more than 100.
- Ninety-two elements are naturally occurring, and the rest are manmade.
- Majority of the elements are solid.
- Eleven elements are in gaseous state at room temperature.
- Two elements are liquid at room temperature — mercury and bromine.
- Elements, gallium and caesium become liquid at a temperature slightly above room temperature (303 k).
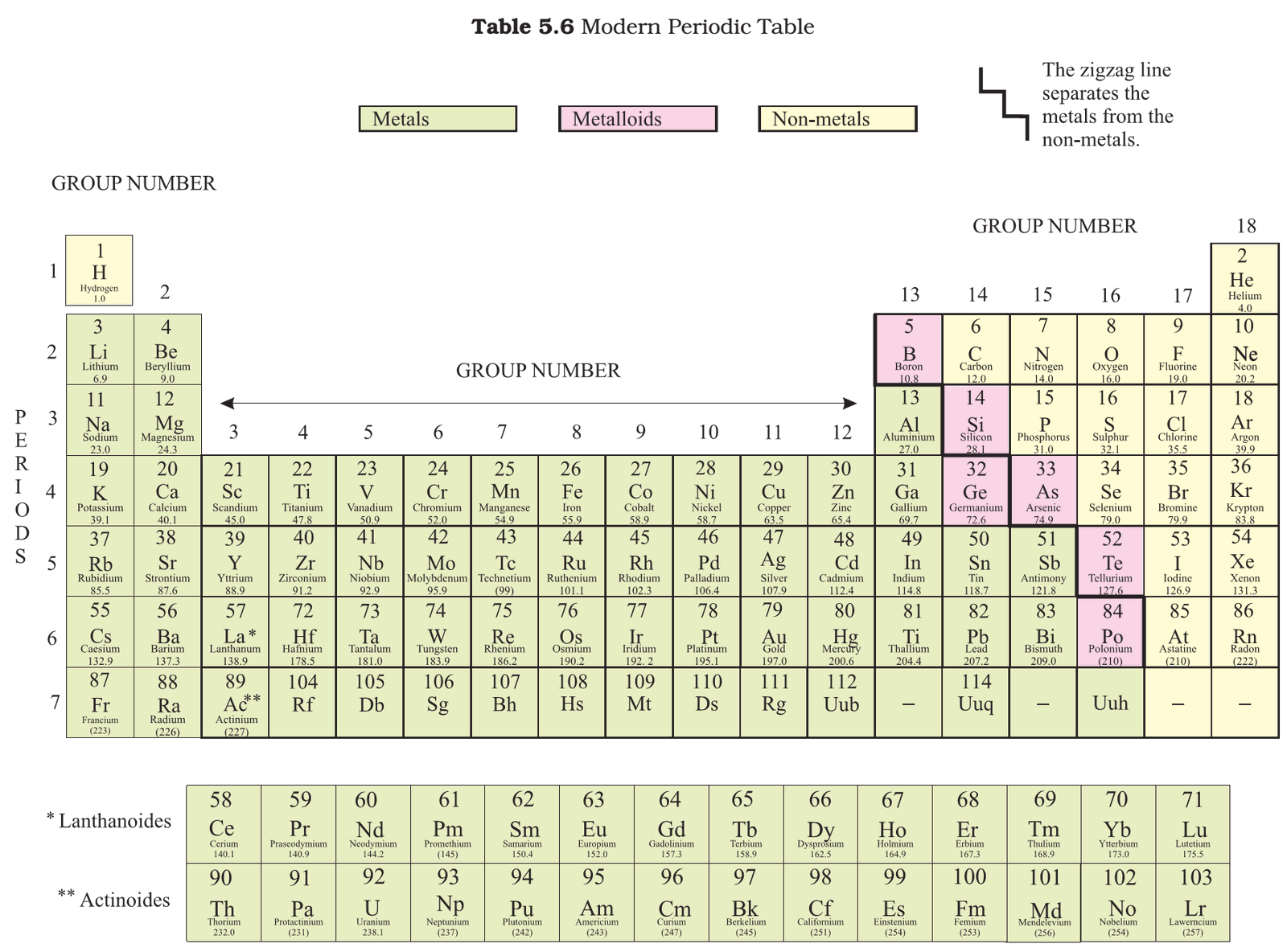
Metals
- They have a lustre (shine).
- They have silvery-grey or golden-yellow colour.
- They conduct heat and electricity.
- They are ductile (can be drawn into wires).
- They are malleable (can be hammered into thin sheets).
- They are sonorous (make a ringing sound when hit)
- Examples of metals are gold, silver, copper, iron, sodium, potassium etc.
- Mercury is the only metal that is liquid at room temperature
Non-metals
- They display a variety of colours.
- They are poor conductors of heat and electricity.
- They are not lustrous, sonorous or malleable.
- Examples of non-metals are hydrogen, oxygen, iodine, carbon (coal, coke), bromine, chlorine etc.
Metalloids
- Some elements have intermediate properties between those of metals and non-metals, they are called metalloids. The six commonly recognised metalloids are:
- Boron
- Silicon
- Germanium
- Arsenic
- Antimony
- Tellurium
Compound
- A compound is a substance formed when two or more chemical elements are chemically bonded together.
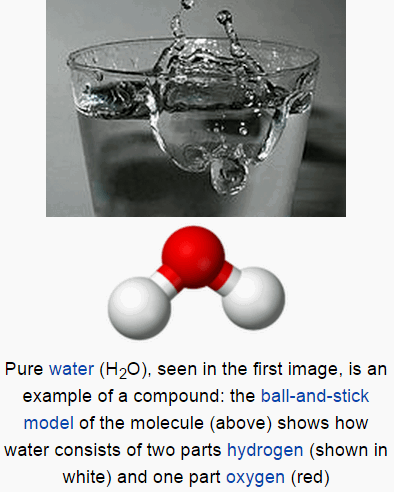
- Two types of chemical bonds common in compounds are covalent bonds and ionic bonds.
- The elements in any compound are always present in fixed ratios.
- A chemical compound can be separated into simpler substances by chemical reactions.
- On heating the two elements strongly we get a compound, which has totally different properties compared to the combining elements.
- The composition, texture and the colour of the compound are the same throughout.
- Properties of a compound are different from its constituent elements, whereas a mixture shows the properties of its constituting elements or compounds.
- Any material that is not a mixture, is called a pure substance.
- Pure substances can be elements or compounds.
Mixtures vs Compounds
- Mixtures are constituted by more than one kind of pure form of matter, known as a substance.
- A substance cannot be separated into other kinds of matter by any physical process.
- Differences between homogeneous and heterogeneous mixtures
Mixtures |
Compounds |
|
|
|
Homogeneous mixtures |
Heterogeneous mixtures |
|
|
|
|
|
|
Source | Credits | Picture Credits: NCERT General Science





![PMF IAS Environment for UPSC 2022-23 [paperback] PMF IAS [Nov 30, 2021]…](https://pmfias.b-cdn.net/wp-content/uploads/2024/04/pmfiasenvironmentforupsc2022-23paperbackpmfiasnov302021.jpg)




Good