Tuberculosis, MDR-TB, National TB Elimination Programme
Subscribe to Never Miss an Important Update! Assured Discounts on New Products!
Must Join PMF IAS Telegram Channel & PMF IAS History Telegram Channel
- Worldwide, tuberculosis (TB) has surpassed HIV-AIDS as the leading cause of death due to infectious diseases.
- India contributes to 27% of the global TB burden, the highest share globally.
- As per WHO, an estimated 4.5 lakh people have MDR-TB and nearly 37,500 people have XDR-TB.
- Out of these, India has 24% of MDR-TB cases in the world.
Tuberculosis (TB)
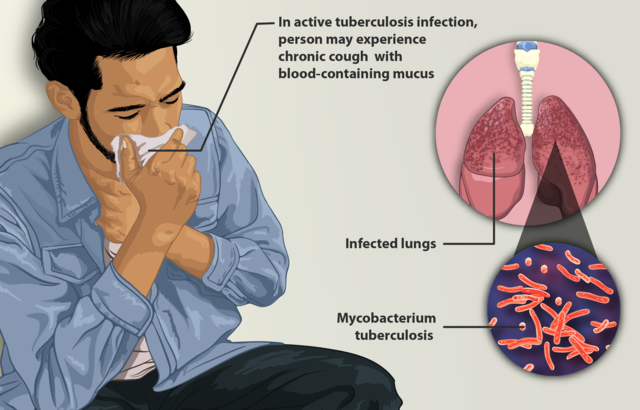
- Tuberculosis (TB) is an infectious disease caused by Mycobacterium tuberculosis.
- TB commonly affects the lungs (pulmonary TB) but can also affect other parts (extrapulmonary TB).
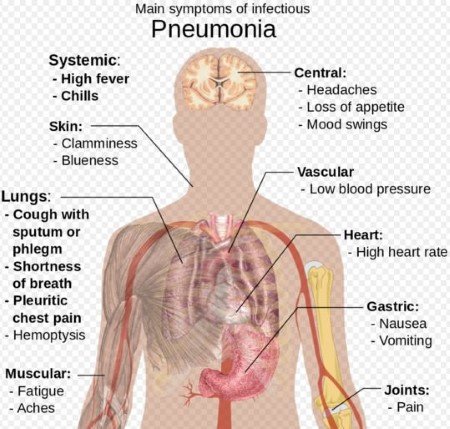
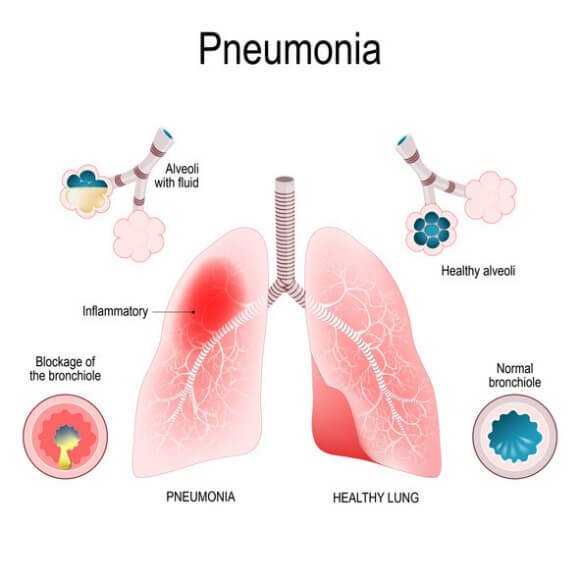
- Pulmonary tuberculosis is a chronic consumptive disease, but it can be present as acute pneumonia.
- Pneumonia is an inflammatory condition of the lung affecting primarily the microscopic air sacs known as alveoli.
- Tuberculosis spreads from person to person through the air, when people who are infected with TB infection cough, sneeze or otherwise transmit respiratory fluids through the air.
- The most common risk factor associated with TB is HIV & other conditions that impair the immune system.
Tuberculosis Symptomatic Diagnosis
- Most infections do not have symptoms, known as latent tuberculosis.
- About 10% of latent infections eventually progresses to active disease which, if left untreated, kills about half of those infected.
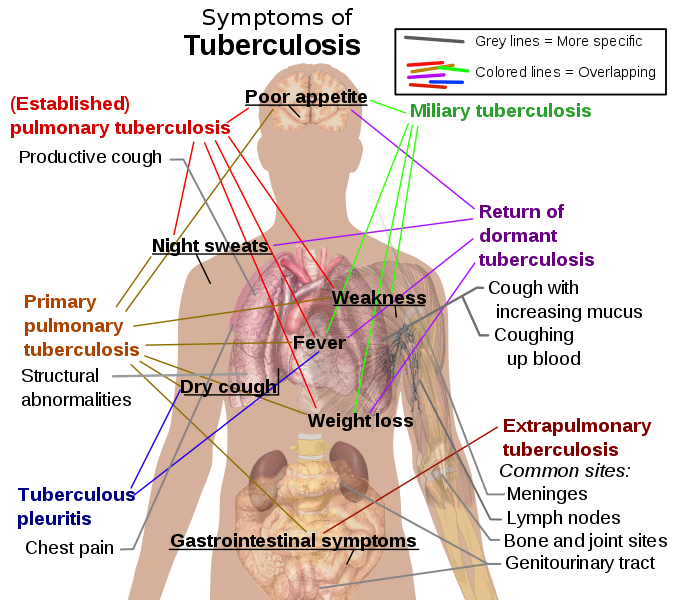
Common symptoms of tuberculosis are:
- Chronic cough with blood-tinged sputum,
- Loss of weight,
- Loss of appetite,
- Fever and night sweats,
- Fatigue , etc.
TB Treatment
- The standard 6-month course treatment consists of 2 phases:
- 1st phase – It lasts for 2 months and is called Intensive phase.
- 2nd phase – It lasts for 4 months and is called Continuous phase.
- For new TB cases, the treatment in intensive phase (IP) consists of four drugs:
- Isoniazid (INH),
- Rifampicin,
- Pyrazinamide &
- Ethambutol.
- For previously treated cases of TB, the intensive phase is of 12 weeks, where injection streptomycin is given for eight weeks along with four drugs.
- Most people with TB are cured by a strictly followed 6-month drug regimen.
Multidrug-Resistant TB (MDR-TB)
- CBNAAT (Cartridges Based Nucleic Acid Amplification Test) is used for early diagnosis of MDR-TB.
- In MDR-TB, the bacteria that cause TB develop resistance to antimicrobial drugs used to cure the disease.
- MDR-TB does not respond to at least isoniazid and rifampicin, the 2 most powerful anti-TB drugs.
- Treatment options for MDR-TB are limited and expensive.
- In some cases, even more severe drug-resistant TB may develop.
Extensively Drug-Resistant TB (XDR-TB)
- XDR-TB is a form of multidrug-resistant TB with additional resistance to more anti-TB drugs.
- People who are resistant to isoniazid and rifampicin, plus any fluoroquinolone and at least one of three injectable second-line drugs (amikacin, kanamycin, capreomycin) are said to have XDR-TB.
Causes of Multidrug Resistant-TB
- Multidrug resistance is caused due to mismanagement of treatment & person-to-person transmission.
- Mismanagement of TB treatment involves inappropriate or incorrect use of antimicrobial drugs or use of ineffective formulations of drugs and premature treatment interruption.
Treatment for Drug-Resistant TB
- The treatment success in MDR-TB patients is about 54%, while it is just 30% in the case of XDR-TB patients.
- A combination of eight drugs for more than a year is need for XDR-TB treatment.
- Treatment success in XDR-TB patients depends on the extent of the drug resistance, the severity of the disease, whether the patient’s immune system is weakened, and adherence to treatment.
- Drugs used for treating MDR-TB and XDR-TB can cause serious adverse effects such as deafness.
New Promising Drug Pretomanid
- Treating MDR-TB and XDR-TB could get simpler and shorter with the new drug Pretomanid.
- Pretomanid is only the third anti-TB drug approved by U.S. FDA in more than 40 years.
- The drug was developed and tested by New York-based non-profit organisation TB Alliance.
What makes Pretomanid promising?
- The duration of treatment for drug-resistant TB can be cut from 18-24 months to just six-nine months when three-drug regimen consisting of Bedaquiline, Pretomanid and Linezolid (BPaL regimen) is used.
- The all-oral, three-drug regimen can vastly improve the treatment success rate & adherence to treatment.
- Importantly, the regimen was found to be safe and effective in curing TB in people living with HIV.
- Unlike bedaquiline, which is expensive, pretomanid might become affordable.
Which category of drug-resistant TB patients will benefit from this new drug?
- BPaL regimen is meant for treating adults with XDR-TB.
- In the case of MDR-TB, the three-drug regimen containing pretomanid can be used only in those patients who cannot tolerate the MDR-TB treatment or do not respond to standard MDR-TB treatment regimen.
- The three-drug regimen is meant only for treating pulmonary TB and should not be used for treating extra-pulmonary TB, drug-sensitive or latent TB.
What are the adverse reactions caused by the drug?
- The three-drug regimen was reported to have caused adverse reactions including liver toxicity (hepatotoxicity), suppression of bone marrow leading to reduced production of red & white blood cells & platelets, etc.
The goal to end TB by 2025
- Revised National TB Control Programme was renamed as the National TB Elimination Programme (NTEP).
- The change in name is in line with the larger goal of eliminating the disease by 2025, five years ahead of the Sustainable Development Goals target of 2030.
National TB Elimination Programme (NTEP)
- 1962: The National TB Programme (NTP) was launched by GOI with BCG vaccination at the district level.
- 1993: WHO declared TB as a global emergency and devised the directly observed treatment (DOTS).
- 1993: GOI revitalized NTP as Revised National TB Control Programme (RNTCP).
- 1997: DOTS was launched as the RNTCP strategy. By 2006 the entire country was covered under RNTCP.
- In its second phase (2006–11), RNTCP improved the quality and reach of services.
- Despite the measures, undiagnosed and mistreated cases continued to drive the TB epidemic.
- A large number of MDR-TB cases were reported every year.
- To address this, National Strategic Plan for Tuberculosis Control 2012-2017 was documented with the goal of ‘universal access to quality TB diagnosis and treatment’.
- Significant interventions were taken during NSP 2012-2017 in terms of mandatory notification of all TB cases, integration of the programme with the National Health Mission, etc.
- To eliminate TB in India by 2025, National Strategic Plan for Tuberculosis Elimination 2017-2025 involving all the stakeholders was formulated by RNTCP.
- On 01-01-2020, RNTCP was renamed as National TB Elimination Programme (NTEP).
National strategic plan for tuberculosis elimination (NSP) 2017-2025 (NSP)
- TB elimination has been integrated into the four strategic pillars of “Detect – Treat – Prevent – Build” (DTPB).
Detect
- Early diagnosis and treatment of TB is an important step in TB elimination.
- The objective of NSP was to find all drug sensitive TB cases (DS-TB) and drug resistant TB cases (DRTB).
- To facilitate TB notification, RNTCP has developed a TB surveillance system called “NIKSHAY” (https://nikshay.gov.in) for both government and private health care facilities.
- For TB diagnosis more than 14,000 designated microscopy centres spread across the country.
- Cartridge Based Nucleic Acid Amplification Tests (CBNAAT) / Line Probe Assay (LPA) has been established at district levels for decentralised molecular testing for drug resistant TB.
- From 2020, GOI will be using Truenat test as a part of early-stage diagnosis.
Treat
- Screening of all patients for rifampicin resistance (and for additional drugs wherever indicated) is done.
- For drug sensitive TB, daily fixed dose combinations (FDCs) of first-line anti-tuberculosis drugs is given.
Prevent
- Isoniazid Preventive Therapy (IPT) is given to children who are close contacts of a TB patient.
- BCG vaccination is provided at birth or as early as possible till one year of age.
- BCG vaccine has a protective effect against meningitis and disseminated TB in children.
Build
- Health system strengthening for TB control under the NSP 2017-2025 is recommended in the form of building and strengthening enabling policies, empowering institutions, and human resources.
Paid users of Science & Technology Notes can download the above content from Sci & Tech May 2021 Current Affairs