Acid Rain & Ocean Acidification (Effects of Air Pollution)
Subscribe to Never Miss an Important Update! Assured Discounts on New Products!
Must Join PMF IAS Telegram Channel & PMF IAS History Telegram Channel
Last updated on April 17, 2024 7:34 PM
- Air Pollution: Air Pollutants, Classification of Air Pollutants (Previous Post)
- Effects of Air Pollution: Acid Rain and Ocean Acidification (This Post)
- Controlling Air Pollution: Bharat Stage VI, National Air Quality Index (Next Post)
Effects of Air Pollution: Acid Rain – Acidification
- Acid rain refers to any precipitation (rain, fog, mist, snow) that is more acidic than normal (pH of less than 5.6. pH below 7 is acidic).
- Acid rain is caused by atmospheric pollution from acidic gases such as sulphur dioxide and oxides of nitrogen emitted from the burning of fossil fuels.
- It is also recognized that acidic smog, fog, mist, move out of the atmosphere and settle on dust particles which in turn accumulate on vegetation as acid depositions.
- When rain falls, the acid from these depositions leak and form acid dews.
The pH scales
- The pH scale is a measure of how acidic or basic (alkaline) a solution is.
- It ranges from 0 to 14. A pH of 7 is neutral.
- A pH less than 7 is acidic, and a pH greater than 7 is basic.
- It is based on hydrogen ion concentration in an aqueous solution.
- pH values decrease as hydrogen ion levels increase.
- A solution with pH 4 is ten times more acidic than pH 5, and a hundred times more acidic than pH 6.
- Whilst the pH range is usually given as 0 to 14, lower and higher values are theoretically possible.
Gases that cause acid rain
| Acidic gases | Source |
| SOx (Sulphur oxides) |
|
| NOx (Nitrogen oxides – NO, NO2 and N2O) |
|
Q. Acid rain is caused by the pollution of the environment by
- carbon dioxide and nitrogen
- carbon monoxide and carbon dioxide
- ozone and carbon dioxide
- nitrous oxide and sulphur dioxide
Explanation:
- CO and CO2 react with rainwater to form weak carbonic acid. Hence, rainwater is naturally slightly acidic. But this is not enough to call it acid rain (acid rain must have pH of less than 5.6).
- Even increased concentration of CO and CO2 is not enough to cause rainwater of pH less than 5.6.
- Only NIOS (10.3.2 Gaseous pollutants > Table 10.3 – Page 167) mentions N2O (nitrous oxide).
More details:
- N2O and NO are neutral in nature.
- N2O3, NO2 and N2O5 are acidic in nature.
- These acidic oxides react with water and produce acids like HNO3 (nitric acid) and HNO2 (nitrous acid) which causes acid rain.
- The neutral oxides are comparatively less, and they combine with oxygen and produce nitrogen dioxide.
- Thus, N2O and NO are indirectly involved (2NO +O2 —>2NO2) in causing acid rain.
Answer: d) nitrous oxide (laughing gas) and sulphur dioxide
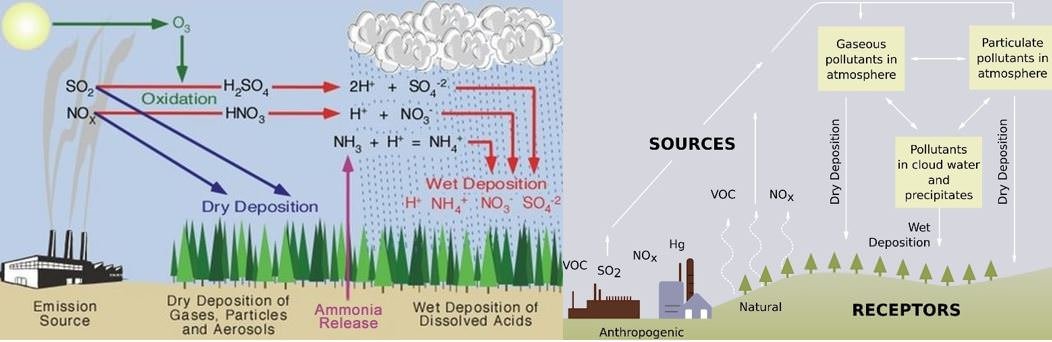
Types of Acid Deposition
- “Acid rain” is a broad term referring to a mixture of wet and dry deposition (a form of deposition material) from the atmosphere.
Wet Deposition
- If the acid chemicals in the air are blown into areas where the weather is wet, the acids can fall to the ground in the form of rain, snow, fog, or mist.
- As this acidic water flows over and through the ground, it affects a variety of plants and animals.
Dry Deposition
- In areas where the weather is dry, the acid chemicals may become incorporated into dust or smoke and fall to the ground through dry deposition, sticking to the ground, buildings, vegetation, cars, etc.
- Dry deposited gases and particles can be washed from these surfaces by rainstorms, through runoff. This runoff water makes the resulting mixture more acidic.
- About half of the acidity in the atmosphere falls back to earth through dry deposition.
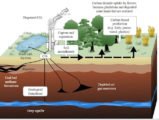
Chemistry of Acid Rain
Six basic steps are involved in the formation of acid rain:
- The atmosphere receives oxides of sulphur and nitrogen from natural and human-made sources.
- Some of these oxides fall back directly to the ground as dry deposition, either close to the place of origin or some distance away.
- Sunlight stimulates the formation of photo-oxidants (such as ozone) in the atmosphere.
- These photo-oxidants interact with the oxides of sulphur and nitrogen and other gases (like NH3) to produce H2SO4 (sulphuric acid) and HNO3 (nitric acid) by oxidation.
- Acid rain containing ions of sulfate, nitrate, ammonium and hydrogen falls as wet deposition.
Harmful effects of acid rain
- Acid precipitation affects both aquatic and terrestrial organisms.
- It also damages buildings and monuments.
Effects on humans
- Bad smells, reduced visibility; irritation of the skin, eyes and the respiratory tract.
- Some direct effects include chronic bronchitis, pulmonary emphysema and cancer.
Effects on soil
- The exchange between hydrogen ions and the nutrient cations like potassium and magnesium in the soil cause leaching of the nutrients, making the soil infertile.
- An increase in ammonia in the soil due to a decrease in other nutrients decrease the rate of decomposition. The nitrate level of the soil is also found to decrease.
- The impact of acid rain on soil is less in India; because Indian soils are mostly alkaline, with good buffering ability.
Effects on aquatic life
- Eggs or sperms of fish, frogs and other aquatic organisms are sensitive to pH changes.
- Acid rain kills their gametes affecting the life cycles and productivity (ecosystem imbalances).
- Acidic lake waters may kill microbes and turn them unproductive.
- Acid rain can make metals bound on soils to be released into the aquatic environment.
Effect on terrestrial life
- Acid rain damage cuticle of plant leaves and reduces photosynthesis.
- Acidic medium promotes leaching of heavy metals like aluminium, lead and mercury. Such metals when percolate into ground water affect soil micro flora/fauna.
- Other indirect effects of acid rain on wildlife are loss or alteration of food and habitat resources.
Effects on microorganisms
- pH determines the proliferation of any microbial species.
- The optimum pH of most bacteria and protozoa is near neutrality.
- Most fungi prefer an acidic environment.
- Most blue-green bacteria prefer an alkaline environment.
- So, microbial species in the soil and water shift from bacteria-bound to fungi-bound.
- This causes a delay in the decomposition of soil organic material.
Effect on buildings, monuments and materials
- Many old, historical, ancient buildings and works of art/textile etc. are adversely affected by acid rain.
- Limestone and marble are destroyed by acid rain. Smoke and soot cover such objects. They slowly dissolve/flake away from the surfaces because of acid fumes in the air.
- Many buildings/monuments such as Taj Mahal in Agra have suffered from acid rain (Marble Cancer).
Acid Rain Areas
- They are concentrated in the industrialised belt of the northern hemisphere.
- Scandinavia, Canada, the Northeast United States and North-western Europe.
In India
- In India, the first report of acid rain came from Bombay in 1974.
- Instances of acid rain are being reported from metropolitan cities.
- Lowering of soil pH is reported from north-eastern India, coastal Karnataka and Kerala, parts of Orissa, West Bengal and Bihar.
Acid Rain Control
- Use of low sulphur fuel or natural gas or washed coal (chemical washing of pulverised coal) in thermal plants can reduce incidences of acid rain.
- Buffering: the practice of adding a neutralising agent to the acidified water to increase the pH. Usually, lime in the form of calcium oxide and calcium carbonate is used.
Ocean Acidification
- Ocean acidification has been called the “evil twin of global warming” and “the other CO2 problem”.
- Ocean acidification is the ongoing decrease in the pH of the Earth’s oceans, caused by the uptake of carbon dioxide (CO2) from the atmosphere.
- An estimated 30–40% of the carbon dioxide from human activity released into the atmosphere dissolves into oceans, rivers and lakes.
- To achieve chemical equilibrium, some of it reacts with the water to form carbonic acid.
- Some of these extra carbonic acid molecules react with a water molecule to give a bicarbonate ion and a hydronium ion, thus increasing ocean acidity (H+ ion concentration).
- Checking CO and CO2 emissions and controlling pollution are the only means to reduce ocean acidification.
Other contributors
- Eutrophication leads to large plankton blooms, and when these blooms collapse and sink to the sea bed the subsequent respiration of bacteria decomposing the algae leads to a decrease in seawater oxygen and an increase in CO2 (a decline in pH).
Effects of Ocean Acidification
- Oceans are an important reservoir for CO2, absorbing a significant quantity of it (one-third) produced by anthropogenic activities and effectively buffering climate change.
- The uptake of atmospheric carbon dioxide is occurring at a rate exceeding the natural buffering capacity of the oceans.
- Increasing acidity depresses metabolic rates and immune responses in some organisms.
- Seawater absorbs CO2 to produce carbonic acid, bicarbonate and carbonate ions.
- However, the increase in atmospheric CO2 levels lead to a decrease in pH level, an increase in the concentration of carbonic acid and bicarbonate ions, causing a decrease in the concentration of carbonate ions.
- The decrease in the amount of carbonate ions available makes it more difficult for marine calcifying organisms, such as coral (calcareous corals) and some plankton (calcareous plankton), to form biogenic calcium carbonate.
- Commercial fisheries are threatened because acidification harms calcifying organisms which form the base of the Arctic food webs.
- Increasing acidity accentuates coral bleaching as corals are very sensitive to changes in water composition.
Impact of Ocean Acidification on Cloud Formation
- The majority of sulphur in the atmosphere is emitted from the ocean, often in the form of dimethylsulfide (DMS) produced by phytoplankton.
- Some of DMS produced by phytoplankton enters the atmosphere and reacts to make sulphuric acid, which clumps into aerosols, or microscopic airborne particles.
- Aerosols seed the formation of clouds, which help cool the Earth by reflecting sunlight.
- But, in acidified ocean water, phytoplankton produces less DMS.
- This reduction of sulphur may lead to decreased cloud formation, raising global temperatures.
Artificial Cloud seeding
|
Q. The acidification of oceans is increasing. Why is this phenomenon a cause of concern?
- The growth and survival of calcareous phytoplankton will be adversely affected.
- The growth and survival of coral reefs will be adversely affected.
- The survival of some animals that have phytoplanktonic larvae will be adversely affected.
- The cloud seeding and formation of clouds will be adversely affected.
Which of statements given above is / are correct?
- 1, 2 and 3 only
- 2 only
- 1 and 3 only
- 1, 2, 3 and 4
Explanation:
We have already learnt that ocean acidification decreases the calcifying ability of corals, calcareous plankton, crustaceans etc. It also adversely affects cloud formation and cloud seeding. So, Options 1, 2 and 4 are correct.
Answer: d) 1, 2, 3 and 4
Last updated on April 17, 2024 7:34 PM











Sir Nitrous Oxide (N₂O) doesn’t cause acid rain so answer for question number 1 would be option b.